
Barastraat 6, 1070 Brussels, Belgium
www.esp-pathology.org
Summary page of the EQA programme
This page provides the information about the programme in a nutshell. It is as concise as it can be.
The programme is focused on external quality assessment of the PD-L1 staining of 4 carcinoma types (subschemes TNBC, NSCLC, UC and HNSCC).
Complete description you can find in the EQA Plan 2024 (namely page 31).
Short description is available in the PDL1 2024 flyer.

| European Society of Pathology Quality Assurance Foundation Barastraat 6, 1070 Brussels, Belgium www.esp-pathology.org |
| SEKK s.r.o., EHK Division Arnošta z Pardubic 2605, 530 02 Pardubice, Czech Republic www.sekk.cz |
The laboratories from all countries are welcome to participate in PDL1 programme.
Very simple guide on how to order the Programmed Death Ligand 1 (PDL1) and Programmed Death Ligand 1 (PDL1) programmes is available here.
The participants are free to order aby combination of the carcinoma types (subschemes).
Each participant examines 1 physical slide and 1 virtual slide for each carcinoma type they ordered.
The table shows complete collection of the samples:
| Set (subscheme) | Samples in the set | |
|---|---|---|
| Physical slide unstained | Virtual slide stained | |
| 1: TNBC | 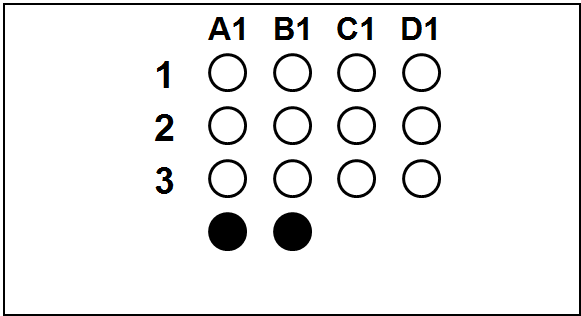
| 
|
| 2: NSCLC | 
| 
|
| 3: UC | 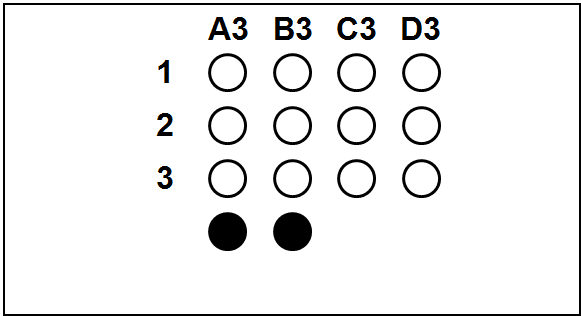
| 
|
| 4: HNSCC | 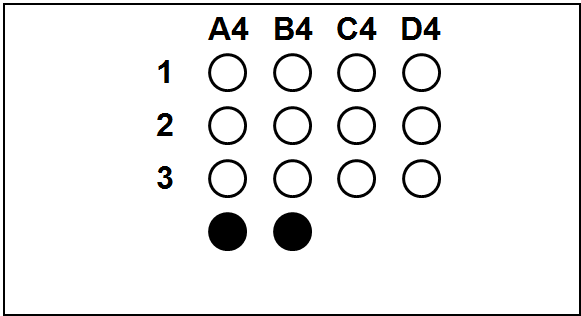
| 
|
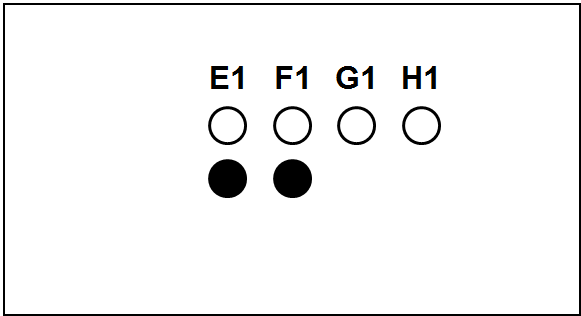
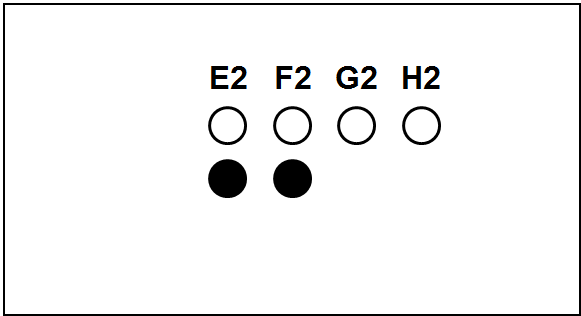
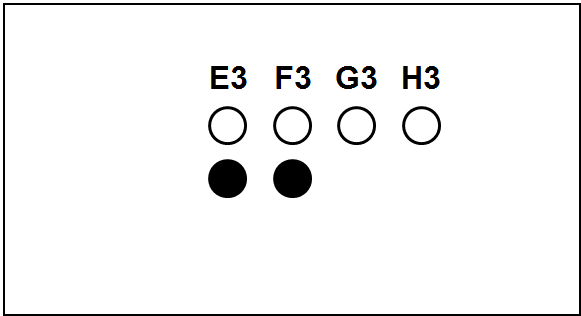
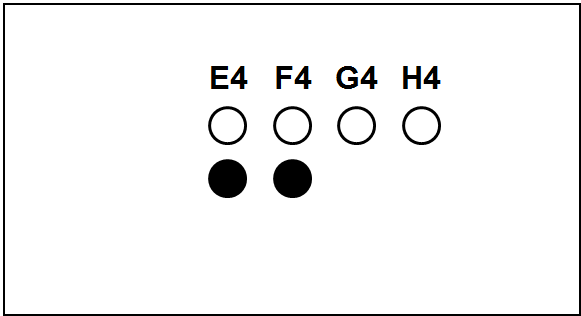
Each slide bears one TMA section.
The columns (A to H) represent individual primary samples (carcinomas) different for each set (thus column A in TNBC slide is a tissue different from the column A in other slides).
There are 3 cores of each primary sample in the physical slide TMA (rows 1 to 3).
Blackened cores (● ●) represent tonsillar tissue (serve as block orientation element and staining quality indicator).
We believe that these examples will give you a good insight.
| Public part of the evaluation (EQA round PDL12/22) | Private part of the evaluation (anonymised and shortened examples from the EQA round PDL12/22) | ||||
 Final report |  Summary statistics |  Confirmation of attendance |  Certificate of approval Certificate of approval
| 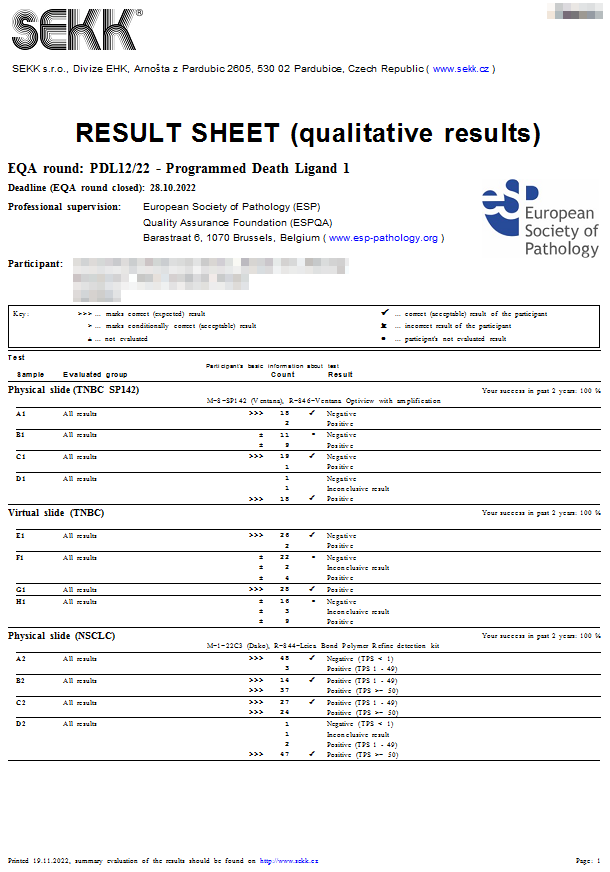 Result sheet Result sheet
| 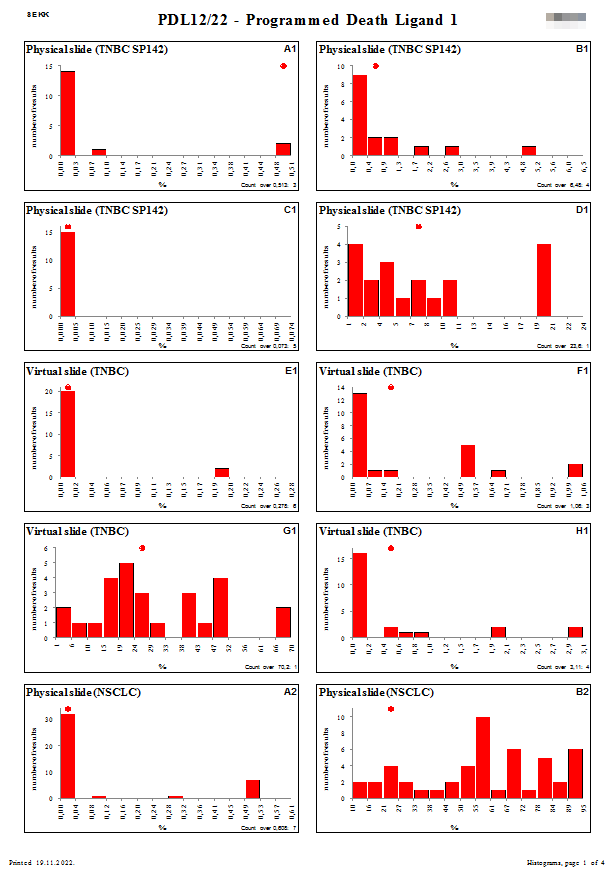 Histograms Histograms
|
To view the latest finished EQA round (PDL12/23) please click here.
Last update: 20.11.2023