
Barastraat 6, 1070 Brussels, Belgium
www.esp-pathology.org
Summary page of the EQA programme
This page provides the information about the programme in a nutshell. It is as concise as it can be.
The programme is focused on external quality assessment of testing of mutations of clinically most important genes
relevant to anti-EGFR therapy in colorectal carcinoma - KRAS, NRAS, BRAF. Participants are expected to identify and describe
mutations, they can choose any combination of KRAS, NRAS, BRAF testing.
Complete description you can find in the EQA Plan 2024 (namely page 30).
Short description is available in the CRC 2024 flyer.

| European Society of Pathology Quality Assurance Foundation Barastraat 6, 1070 Brussels, Belgium www.esp-pathology.org |
| SEKK s.r.o., EHK Division Arnošta z Pardubic 2605, 530 02 Pardubice, Czech Republic www.sekk.cz | Accreditation: | Certificate of accreditation Appendix |
The laboratories from all countries are welcome to participate in CRC programme.
Very simple guide on how to order the Colorectal Carcinoma (CRC) and Programmed Death Ligand 1 (PDL1) programmes is available here.
We believe that these examples will give you a good insight.
| Public part of the evaluation (EQA round CRC1/23) | Private part of the evaluation (anonymised and shortened examples from the EQA round CRC1/23) | |||
 Final report |  Summary statistics |  Confirmation of attendance |  Certificate Certificate
| 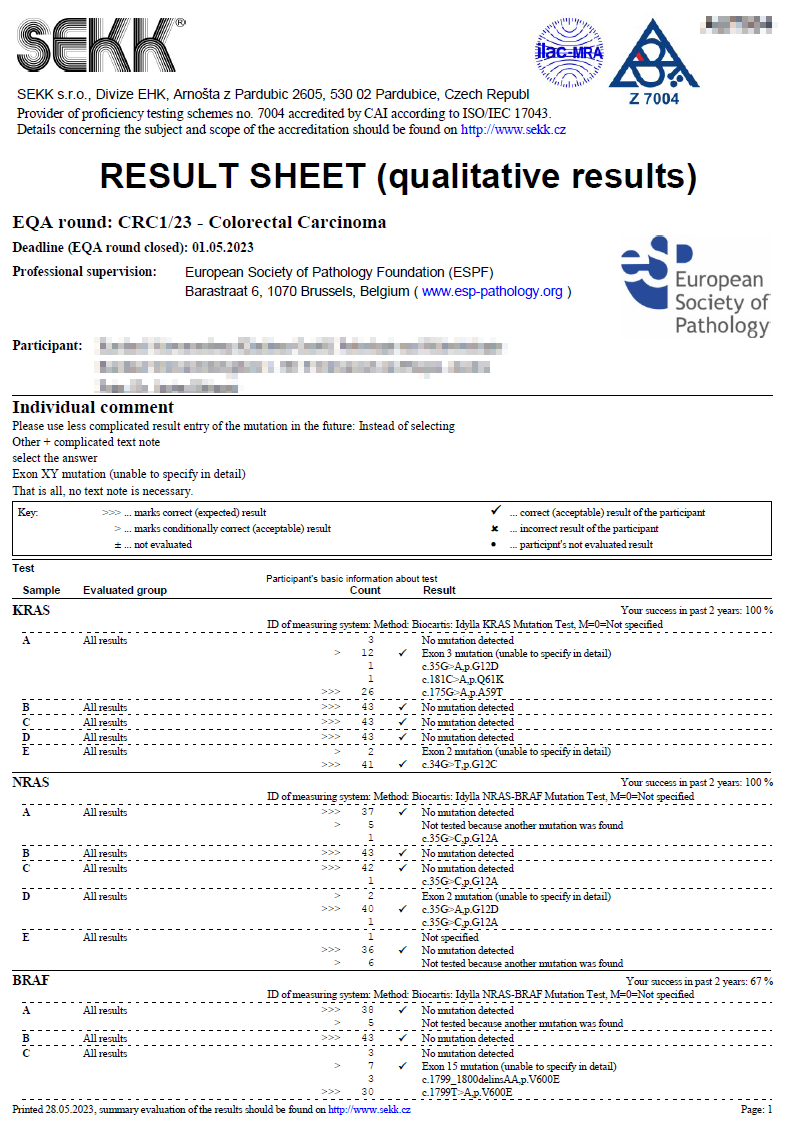 Result sheet Result sheet
|
To view the latest finished EQA round (CRC2/23) please click here.
Last update: 13.10.2023